April 21, 2006
Problems with Pyrex - part two
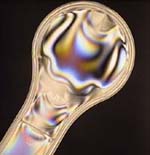
Just a quick note. About a month ago, I had written about how today's Pyrex glass cookware might not be what Pyrex originally was. When Pyrex cookware first came out, it was a true refrigerator to oven to table to refrigerator product. Lately though, people have been experiencing catastrophic failures of their Pyrex cookware when it has been subjected to the most modest changes in temperature or shock. The two things that give Pyrex its properties are a 10% Boron content and a long annealing process after manufacture. From my first entry:#1) - Pyrex is another name for Borosilicate Glass and contains about 10% Boric Oxide in addition to the Silica and other chemicals. From Wikipedia:I do not have the equipment to test for Boron but there is a very simple test to see if a piece of glass has been annealed or not. If it is not annealed, it will have stress and by using a set of crossed polarized filters, I can see and photograph the stress. Here is an example of a piece with internal stresses under regular light and under cross-polarized light:The boron gives borosilicate glass a reduced thermal expansion coefficient (about one-third that of ordinary glass). This reduces material stresses caused by temperature gradients, thus making it more resistant to breaking.
#2) - Pyrex is also annealed which means that after it is molded to shape, it is held at near melting temperatures for a long time to allow the residual stresses to 'mellow out'.
From Wikipedia:Annealing, in glassblowing and lampworking, is heating a piece of glass until its temperature reaches a stress-relief point, that is, a temperature at which the glass is still too hard to deform, but is soft enough for internal stresses to ease. The piece is then allowed to heat-soak until its temperature is even throughout; the time necessary for this varies depending on the type of glass and thickness of the thickest section. The piece is then slowly cooled at a predetermined rate until its temperature is below a critical point, at which it can no longer generate internal stresses, and then the temperature can safely be dropped to room temperature. This relieves the internal stresses, making the glass much more durable. Glass which has not been annealed will crack or even shatter when subjected to a relatively small temperature change or other shock.

Comments
Post a comment