Amphiphilic organosilane-directed synthesis of crystalline zeolite with tunable mesoporosity
Whew... I think I'll stick with The Korea Times version of the story instead of the one at
Nature Materials.
From
The Korea Times:
Korean Scientists Create Magical Substance
A team of Korean scientists has created a new substance that can convert inexpensive intermediate crude oil to valuable gasoline in a very efficient fashion.
The team, led by Prof. Ryoo Ryong at the Korea Advanced Institute of Science and Technology, yesterday said they had generated a new-type of zeolite that can be a petrochemical catalyst dozens of times more effective than today's norm.
A bit more:
"Zeolite is the most widely-used catalyst now in the petrochemical industry. It has many outstanding advantages but the hitch is that its efficiency was mediocre due to its slow reaction rate," Ryoo said.
"In comparison, our new-fangled zeolite shows a substantially advanced reaction rate, at 1.5 times to dozens of times better than the one currently available," the 50-year-old professor said.
Zeolite refers to a group of minerals that have a porous structure. It makes extremely active catalysts by confining molecules in small spaces with its crystalline pores.
In the value-added process of upgrading gas oils to gasoline, firms from around the globe widely resort to the substance as a significant catalyst.
By comparison, here is the title and opening paragraph from the
Nature Materials paper:
Amphiphilic organosilane-directed synthesis of crystalline zeolite with tunable mesoporosity
Zeolites are a family of crystalline aluminosilicate materials widely used as shape-selective catalysts, ion exchange materials, and adsorbents for organic compounds1, 2. In the present work, zeolites were synthesized by adding a rationally designed amphiphilic organosilane surfactant to conventional alkaline zeolite synthesis mixtures. The zeolite products were characterized by a complementary combination of X-ray diffraction (XRD), nitrogen sorption, scanning electron microscopy (SEM) and transmission electron microscopy (TEM). The analyses show that the present method is suitable as a direct synthesis route to highly mesoporous zeolites. The mesopore diameters could be uniformly tailored, similar to ordered mesoporous silica with amorphous frameworks3. The mesoporous zeolite exhibited a narrow, small-angle XRD peak, which is characteristic of the short-range correlation between mesopores, similar to disordered wormhole-like mesoporous materials4, 5. The XRD patterns and electron micrographs of the samples taken during crystallization clearly showed the evolution of the mesoporous structure concomitantly to the crystallization of zeolite frameworks. The synthesis of the crystalline aluminosilicate materials with tunable mesoporosity and strong acidity has potentially important technological implications for catalytic reactions of large molecules, whereas conventional mesoporous materials lack hydrothermal stability and acidity.
An English Major he is not but he is a genius and hopefully soon, a very suce$$ful chemist... Very cool.
We are seeing advances in the
Fischer-Tropsch process.
(I wrote about them
here,
here and
here.); maybe the two will play nice together. F-T is a catalytic process and this new Zeolite allows catalysts to work much faster.
Here is a Scanning Electron Micrograph of the little buggers:
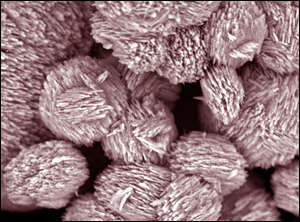
Cute!
Posted by DaveH at August 8, 2006 8:22 PM
| TrackBack
